Standalone, turnkey, process ready cGMP modular cleanrooms
Explore comprehensive insights into our innovative design, ISO compliance, and state-of-the-art equipment
Want to learn more about our trailers?
Download our guide which has all the resources you need to make an informed choice.
Mobile Cleanrooms: A Comprehensive Overview
Stripes Global pioneers with its cutting-edge mobile medical trailers for modern healthcare needs.
Mobile Cleanrooms: A Comprehensive Overview
Stripes Global pioneers with its cutting-edge mobile medical trailers for modern healthcare needs.
In full compliance and turnkey, we follow the principles of QC (Quality Control) and CQV (Commissioning, Qualification, & Validation) throughout our cleanroom manufacturing process to provide a ready-to-use cleanroom suite. Design and engineering controls are application-driven to ensure the cleanroom facility meets in-process validation checks and facility EMPQ requirements.
Our platform product offers a pre-installation consultation and assigns a dedicated project manager to serve as your single point of contact from project initiation through site installation, preventative maintenance, and facility monitoring.
Designed for efficient usp 797 sterile compounding
Serviced & maintained by Stripes Global
Freestanding outside in any region
Connects to existing operations
Integrated HVAC & MEP
Rapidly movable and made for Immediate use
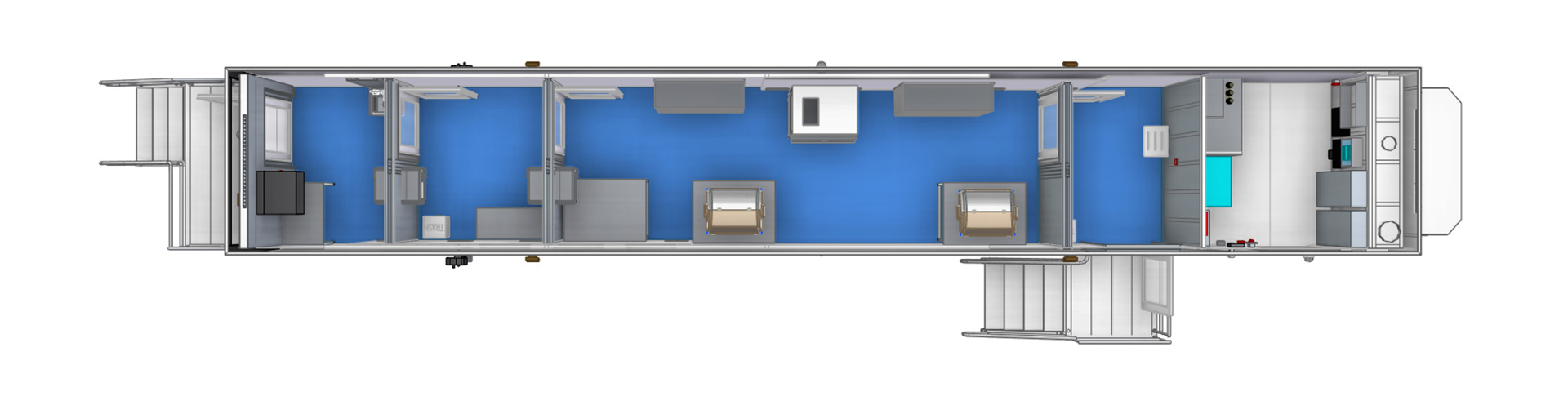
Mobile cGMP Cleanrooms
Designed for a variety of cGMP applications and workflows. This bidirectional flow layout provides the maximum manufacturing space for cGMP operations. This manufacturing facility has a Grade C (ISO 7) cleanroom classification with adjoining anteroom at GRADE D/C (ISO 8/7). This facility is ideal for small scale manufacturing for cell and gene therapy applications using a distributed or point-of-care manufacturing model.
Talk to us about mobile compounding pharmacies today
Specifications
Security, Access, Fire & Data
- CCTV with 1TB storage system
- 4 internal cameras
- 4 external cameras
- Fire & burglar alarm
- Unique code personnel access control
- Intercom system for in-lab communication
- Optional: Key Card Access
Plumbing
- Ready to accept site infrastructure for both fresh and grey water.
- Onboard hand wash sink with hot water
- Optional: Purified water
Environmental Monitoring System
- Monitors critical environmental conditions, trends and stores environmental data. FDA cGMP Part 11 compliant.
- In lab user interface
Building Management System
- Reliable controls central HMI, remote access (requires client IT interface), includes calibrated sensors for parameter control & monitoring, audible & visible alarm in each space
Electrical
- 208/120V 3-phase, 225amp service
- Optional: Generator or Battery Back-Up Power
Door Controls
- Wave to open door controls with configurable interlocks and timing
Mechanical
- Stand-alone, fully integrated HVAC, built to maintain critical environmental conditions required for cGMP manufacturing.
- HVAC sized to optimize Air Changes per Hour (ACH) rates
- Low wall returns create a particle reducing air turnover
- Temperature, Humidity, & Differential Pressure Controls
- Robust HVAC allows switching between positive & negative pressure for BSL-2 containment.
- Designed for reliability in varying climates & regions
Architectural
- Stainless Steel, Powder coated or FRP Doors.
- Drop or Monolithic Arcoplast ceiling with Coved flooring.
- Facility is designed for cleanability and chemical compatibility
Integrated Equipment
- Active, HEPA-purging 100% stainless steel material pass through for an optimized material flow.
- 100% stainless steel casework, designed for mobile applications with optimal cleanability & durability.
- Class 2 BSC or Laminar Flow Workstation
- Optional: Gowning benches, shelving & storage
Medical Trailer Excellence
At Stripes Medical, we seamlessly blend mobility with both USP 797 and USP 800 compounding precision. Our cleanroom medical trailers offer the unparalleled convenience of transportability without compromising on accuracy and quality. For those seeking the best in mobile healthcare, Stripes Medical delivers excellence in every journey
Beyond Standards
Stripes Global pioneers with its cutting-edge mobile medical trailers for modern healthcare needs.
At Stripes Medical, we don’t just adhere to the FDA’s cGMP regulations – we aim higher. Our mobile medical trailers are designed not only to meet mobile pharmacy solutions but to surpass industry standards. With advanced features and cutting-edge technology, we’re setting new benchmarks for mobile healthcare. Choose Stripes, where excellence is our standard.
Expert Insights at Your Fingertips
Stripes Global pioneers with its cutting-edge mobile medical trailers for modern healthcare needs.
Your 7-Step Guide to Medical Device Manufacturing
Have an idea for medical device manufacturing? A new surgical, dental or life science device that will either, 1. Revolutionize a procedure or system; or 2. At a minimum, make life easier for...
The Sterilization Problem
You'll make a big mistake if you think sterilization management only relates to medical device manufacturing and development logistics. On the contrary, sterilization management is one of the final...
Designing Small Medical Devices for Smart Tech and Wearables
Tech small devices are generally getting smarter, and the medical niche is not an exception to this advancement. Therefore, designing small medical devices is becoming a priority to improve...


Headquarters
3309 56th St, Ste. 104
Gig Harbor, WA 98335
Manufacturing and Hubzone Location
604 Pottawatomie St.
Leavenworth, KS 66048
DUNS Number: 080204009 | CAGE Code: 7L6E6 | SAM Unique Entity ID: FTVNJZY96AW4
NAICS Codes
325413 | 325414 | 334510 | 334515 | 334516 | 334519 | 339112 | 339113 | 339114 | 339115 | 339115 | 339116 | 423390 | 423450 | 423460 | 423510 | 423840 | 424130 | 425120 | 493110 | 541380 | 339116 | 423390 | 423450 | 423460 | 423510 | 423840 | 424130 | 425120 | 493110 | 541380 | 541512 | 541519 | 541611 | 541612 | 541690 | 541711 | 541712 | 541940 | 541990 | 621111 | 621112 | 621511