Mobile Compounding Pharmacies: USP 797 & 800 Compliance Delivered On-Site, Anytime
Call now to speak with an expert and ensure safe, efficient pharmaceutical operations.
Want to learn more about our trailers?
Enter your email to instantly receive our free guide to USP 797/800 compliance and trailer features.
Mobile Compounding Pharmacies: Compliance, Flexibility, and Speed at Your Fingertips
Need safe, compliant mobile compounding solutions right now? Stripes Global delivers mobile medical trailers that meet USP 797/800 standards, ensuring you stay operational without any compliance risk.
Mobile Compounding Pharmacies: Compliance, Flexibility, and Speed at Your Fingertips
Need safe, compliant mobile compounding solutions right now? Stripes Global delivers mobile medical trailers that meet USP 797/800 standards, ensuring you stay operational without any compliance risk.
Our mobile compounding units are designed to ensure compliance with USP 797/800 and FDA cGMP regulations, so you can deliver medication quickly and safely. From innovative ISO room classifications to rapid deployment, we provide everything you need to meet your compounding goals with ease.
Designed for efficient compounding throughput
Serviced & maintained by Stripes Global
Freestanding outside in any region
Connects to existing operations
Integrated HVAC & MEP
Rapidly movable and ready for immediate use
Expert Insights at Your Fingertips
Stripes Global pioneers with its cutting-edge mobile medical trailers for modern healthcare needs.
Your 7-Step Guide to Medical Device Manufacturing
Have an idea for medical device manufacturing? A new surgical, dental or life science device that will either, 1. Revolutionize a procedure or system; or 2. At a minimum, make life easier for...
The Sterilization Problem
You'll make a big mistake if you think sterilization management only relates to medical device manufacturing and development logistics. On the contrary, sterilization management is one of the final...
Designing Small Medical Devices for Smart Tech and Wearables
Tech small devices are generally getting smarter, and the medical niche is not an exception to this advancement. Therefore, designing small medical devices is becoming a priority to improve...
Mobile Cleanroom Built for Compliance: USP <797>/<800> Solutions at Your Doorstep
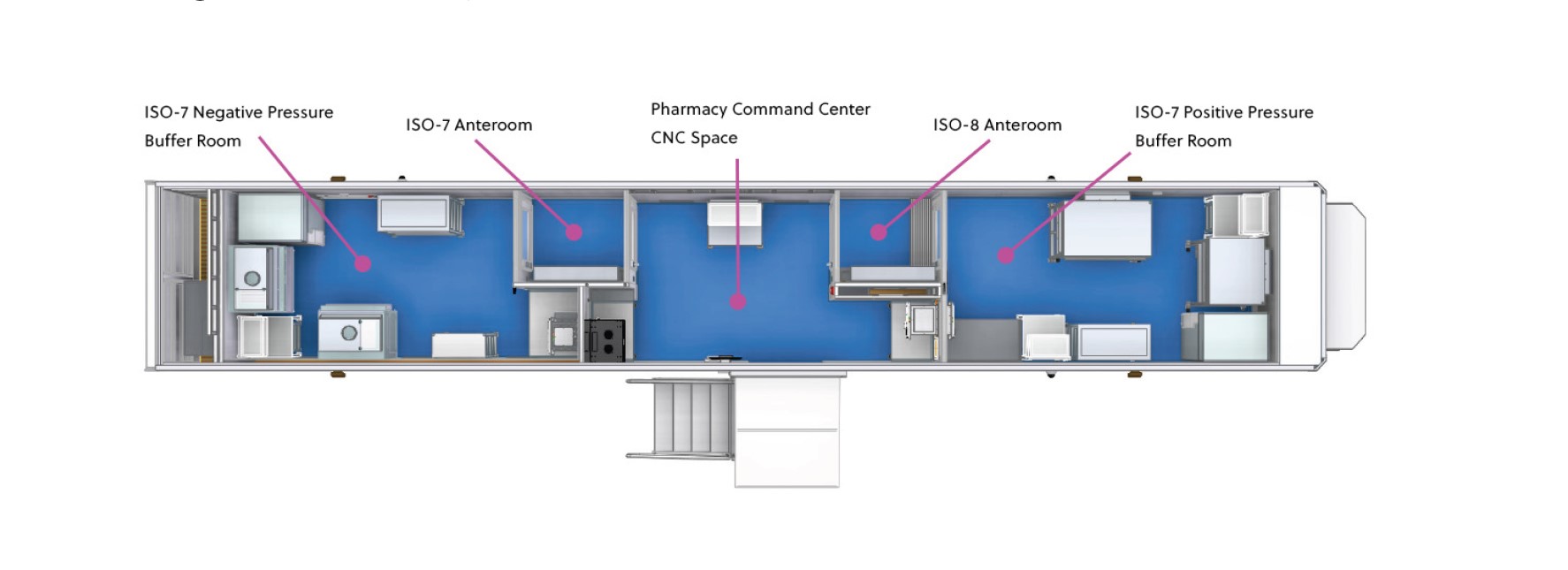
Our 53-foot mobile cleanroom is meticulously designed to meet both non-hazardous (USP <797>) and hazardous (USP <800>) compounding needs. With ISO 7 and ISO 8 buffer rooms, you can trust that every transition within your facility is fully compliant and secure. Ensure safety and operational efficiency, no matter where you deploy.
Need a mobile compounding solution? Call Us Now
Specifications
Room Classification Types
- CNC Entry
- ISO 7 Hazardous Buffer
- ISO 7 Anteroom
- ISO 7 Non-Hazardous Buffer
- ISO 8 Anteroom
- NC Mechanical Room
Building Management System
- A central BMS controls all aspects of the facility via BACnet and can report via BACnet to a centralized facility
Mechanical
- 450 sq ft Mobile Compounding Pharmacy with 4 Class 100 Germfree Laminar Flow Hoods
- Fully integrated HVAC with separate mechanical space
- Our single HVAC system accommodates hazardous and non-hazardous compounding in the same facility
- Easily movable by a standard semi truck
Plumbing
- Internal, domestic water distribution point ready to accept site water connections
- Eyewash in buffer rooms
- Complaint with current International Building Code (IBC)
Electrical
- Trailer is equipped with internal 208V 3- phase electrical connection
- NFPA 70 NEC 2020 Compliant
Architectural
- All finishes are durable against harsh cleaning agents such as sporicidal agents and VHP
- Every build decision prioritizes a sealed, smooth, cleanable and compliant interior envelope
- Seamless 40′ Arcoplast ® walls
Environmental Monitoring System
- Optional: 21 CFR Part 11 EMS system that can serve as permanent record keeping on environment conditions
- Room temperature, relative humidity, room pressure “at rest” and “in operation”, and differential pressures are all captured
Door Controls
- Wave to open door controls with configurable interlocks and timing
- Variety of access control systems can be integrated
Integrated Equipment
- Vertical and Horizontal Laminar Flow Workstations
- Biosafety Cabinets, Class 2 A2 and B2
- Active-HEPA Material Pass Through
Built for Compliance & Efficiency
At Stripes Medical, we seamlessly blend mobility with both USP 797 and USP 800 compounding precision. Our trailers offer the unparalleled convenience of transportability without compromising on accuracy and quality. For those seeking the best in mobile healthcare, Stripes Medical delivers excellence in every journey
Beyond Standards
Stripes Global pioneers with its cutting-edge mobile medical trailers for modern healthcare needs.
At Stripes Medical, we don’t just adhere to the FDA’s cGMP regulations – we aim higher. Our mobile medical trailers are designed not only to meet but to surpass industry standards. With advanced features and cutting-edge technology, we’re setting new benchmarks for mobile healthcare. Choose Stripes, where excellence is our standard.


Headquarters
3309 56th St, Ste. 104
Gig Harbor, WA 98335
Manufacturing and Hubzone Location
604 Pottawatomie St.
Leavenworth, KS 66048
DUNS Number: 080204009 | CAGE Code: 7L6E6 | SAM Unique Entity ID: FTVNJZY96AW4
NAICS Codes
325413 | 325414 | 334510 | 334515 | 334516 | 334519 | 339112 | 339113 | 339114 | 339115 | 339115 | 339116 | 423390 | 423450 | 423460 | 423510 | 423840 | 424130 | 425120 | 493110 | 541380 | 339116 | 423390 | 423450 | 423460 | 423510 | 423840 | 424130 | 425120 | 493110 | 541380 | 541512 | 541519 | 541611 | 541612 | 541690 | 541711 | 541712 | 541940 | 541990 | 621111 | 621112 | 621511